
About Albumin
HUMAN ALBUMIN SOLUTIONS HAVE BEEN USED THERAPEUTICALLY IN THE UNITED STATES FOR MORE THAN 80 YEARS.1,2
Human albumin solution is an extensively studied plasma protein used for a broad range of clinical conditions.3
The key indication for albumin is the restoration and maintenance of circulating blood volume for trauma, surgery, blood loss, hypoalbuminemia, and burns.4,5
The dose requirements are dependent on the size of the patient, severity of trauma or illness, and continuing fluid and protein losses. The degree and duration of volume expansion depends on a patients’ clinical condition and initial circulatory volume, but the effect may be diminished with dehydration.4,5
THERAPEUTIC HUMAN ALBUMIN SOLUTIONS ARE AVAILABLE AS 5% ISOTONIC OR 25% HYPERTONIC SOLUTIONS

ISOTONIC 5% ALBUMIN SOLUTIONS
- Have an osmotic pressure almost identical to that of normal plasma6,7
- Are often used for blood volume replacement1
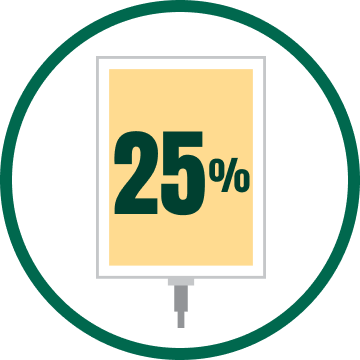
HYPERTONIC 25% ALBUMIN SOLUTIONS
- Have an osmotic pressure greater than plasma6
- Help increase fluid volume by drawing water out of the intercellular space7
- Are often used for blood volume expansion1
GET ANSWERS TO YOUR QUESTIONS.
Takeda has a dedicated and experienced hospital team of account managers who can provide a personal and tailored approach to address the needs of your system.
References
- Marketing Research Bureau. The Plasma Proteins Market in the United States 2019. Published July 2020.
- Center for Biologic Evaluation and Research. List of licensed biological products with reference produce exclusivity and biosimilarity or interchangeability evaluations to date. Accessed February 17, 2025. Available at: https://www.fda.gov/media/105605/download
- Bertolini J, Goss N, Curling J, eds. Production of Plasma Proteins for Therapeutic Use. Hoboken, NJ: John Wiley & Sons; 2013.
- FLEXBUMIN 25% [Albumin (Human)], USP, 25% Solution. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- FLEXBUMIN 5% [Albumin (Human)], USP, 5% Solution. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7(3):216-234.
- Crawford A, Harris H. I.V. fluids: What nurses need to know. Nursing. 2011;41(5):30-38.
