
FLEXBUMIN
Features
SUITABLE FOR HOSPITALS LOOKING FOR EFFICIENCY, STERILITY, AND EASE OF USE1-9
EFFICIENCY AND EASE OF USE
SAVES SHELF SPACE
The FLEXBUMIN container saves space on pharmacy shelves, helping make human albumin solution readily available to HCPs in critical situations.1,2,6-9
- Dispensing cabinets can hold at least 57% more FLEXBUMIN containers than albumin in glass bottles of equal volume9*
FAST, SIMPLE SETUP
FLEXBUMIN setup time was on average 40% faster, taking 43 seconds compared to 66 seconds and 78 seconds for a glass bottle of similar volume, using hangers and sleeves, respectively.8
FLEXBUMIN can be set up in three simple steps—meaning fewer steps in workflow for healthcare professionals and reduced time to administration in the hospital and critical care settings.1,2,8

REDUCED WASTE
The FLEXBUMIN container is designed to reduce disposal volume and overall waste.1,2,5,10,11
- Approximately 90% lighter than empty glass bottles of equal volume7,12†
- Room temperature storage for up to 3 years13
- With no protective overwrap needed, FLEXBUMIN is delivered with no extra packaging
*Calculations were based on an Omnicell dispensing cabinet and comparison of FLEXBUMIN 25% 50 mL and 100 mL containers and FLEXBUMIN 5% 250 mL containers with containers of BUMINATE® [Albumin (Human)], USP 5% and 25% solutions in glass bottles of equal volume.9
†Calculation based on BUMINATE® [Albumin (Human)], USP, 5% and 25% solutions in glass bottles of equal volume.7,12
STERILITY AND SAFETY

FLEXIBLE, SHATTERPROOF CONTAINER
Delivered in a shatterproof, flexible container, FLEXBUMIN may help reduce risk in critical situations.1,2,5-10
CLOSED INFUSION SYSTEM
The FLEXBUMIN container is fully collapsible with self-sealing injection ports. It connects with standard IV administration sets to form a sterile closed infusion system.3,5,10
STERILITY
The FLEXBUMIN container is designed to maintain sterility during setup and administration —without a protective overwrap or requirement to swab the injection port.1-5,10

LATEX FREE
The FLEXBUMIN container is free of latex, PVC, DEHP, and DEHA.14 There is no known risk of aluminum leaching from the container.15
DEHA=diethylhexyl adipate; DEHP=diethylhexyl phthalate; PVC=polyvinyl chloride.
INDICATIONS

FLEXBUMIN 5%, ALBUMIN (HUMAN) SOLUTION IS INDICATED FOR2:
- Hypovolemia
- Hypoalbuminemia: burns
- Cardiopulmonary bypass surgery
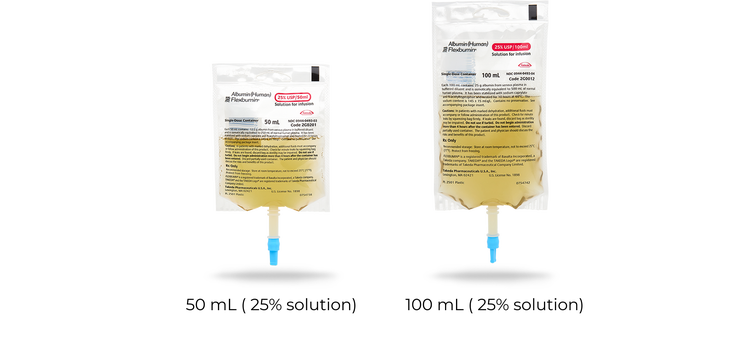
FLEXBUMIN 25%, ALBUMIN (HUMAN) SOLUTION IS INDICATED FOR1:
- Hypovolemia
- Hypoalbuminemia: burns, adult respiratory distress syndrome (ARDS), and nephrosis
- Cardiopulmonary bypass surgery
- Hemolytic disease of the newborn (HDN)
Albumin is not indicated as an intravenous nutrient.
DOSING1,2
FLEXBUMIN 5% Solution and FLEXBUMIN 25% Solution are for intravenous use only.
- Adjust dose and rate of infusion based on the patient’s clinical status
- Do not exceed 2 g of albumin per kg body weight for the daily dose
- Do not exceed 1 mL/min for patients with normal blood volume
- Do not dilute with Sterile Water for Injection

ADMINISTRATION
FLEXBUMIN can be set up in three simple steps—reducing time to administration.1,2,8‡

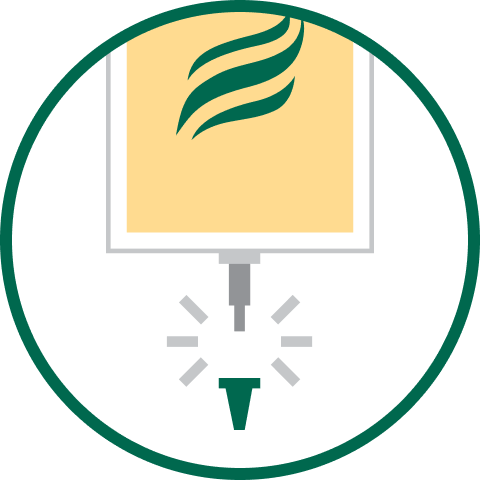
1. HANG
Suspend from eyelet support.
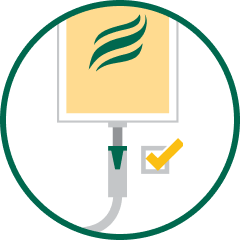
2. UNCAP
Remove plastic protection from outlet.
3. CONNECT
Attach standard administration set.
Thoroughly inspect the flexible container for small leaks, particulate matter, and discoloration prior to administration. Do not use the bag if the tip protector is damaged, detached, or missing.1,2
‡Administration caution: Do not use plastic container in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of fluid from the secondary container is complete.1,2
Y-connector sets enable dual infusion when large volumes of FLEXBUMIN 5% [Albumin (Human)] are required16
- Y-connector sets are compatible with infusion pump tubing spikes and are suitable for gravity administration16
- Five non-DEHP, non-latex Y-connector sets are included in each 10-bag case of FLEXBUMIN 5% when ordered using the Y-connector code17,18
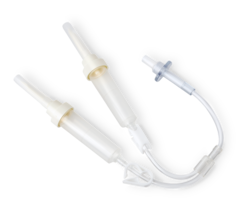
How does the FLEXBUMIN Y-connector work?
Watch the demonstration video below.
STORAGE & HANDLING
Store at room temperature: not to exceed 25 °C (77 °F). Protect from freezing.1,2
Can be stored at room temperature for up to 3 years based on the expiration date.13

GET ANSWERS TO YOUR QUESTIONS.
Takeda has a dedicated and experienced hospital team of account managers who can provide a personal and tailored approach to address the needs of your system.
References
- FLEXBUMIN 25% [Albumin (Human)], USP, 25% Solution. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- FLEXBUMIN 5% [Albumin (Human)], USP, 5% Solution. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- Maki DG, Rosenthal VD, Salomao R, et al. Impact of switching from an open to a closed infusion system on rates of central line-associated bloodstream infection: a meta-analysis of time-sequence cohort studies in 4 countries. Infect Control Hosp Epidemiol. 2011;32(1):50-58.
- The Joint Commission. Preventing Central Line-Associated Bloodstream Infections: A Global Challenge, A Global Perspective. Accessed February 20, 2025. https://www.jointcommission.org/-/media/tjc/documents/resources/hai/clabsi_monographpdf.pdf
- Data on file. VV-MED-7455—FLEX-013 FLEXBUMIN biologics license application.
- Data on file. VV-MED-7220—FLEX-006 Plastic vs glass weights and dimensions.
- Data on file. No 68128FR. GALAXY 250 mL weight comparisons.
- Data on file. FLEXBUMIN time and motion study. March 17, 2010.
- Data on file. No. 688877. GALAXY storage space comparisons. Baxter Healthcare Corporation.
- Data on file. FLEXBUMIN 25% biological license application.
- Data on file. VV-MED-7218—FLEX-004 Albumin stability summary and conclusion.
- Data on file. VV-MED-7242—FLEX-008 FLEXBUMIN GALAXY Container Disposal Cost.
- Data on file. Flexbumin 5% and 25% Shelf Life 082024.
- Data on file. VV-MED-7449 Certificate of nonuse: concerned chemicals for environment.
- Data on file. VV-MED-7449—FLEX-012 Analysis of aluminum levels in FLEXBUMIN during stability.
- Perucca R. Infusion therapy equipment: types of infusion therapy equipment. In: Hankins J, Lonsway RA, Hedrick C, Perdue MB, eds. The Infusion Nurses Society Infusion Therapy in Clinical Practice. 2nd ed. Philadelphia, PA: Saunders; 2001.
- CODAN. Product Catalog. Accessed March 3, 2025. Available at: https://www.codanusa.com/product-catalog/
- US National Library of Medicine. Access GUDID: CODAN (10813153024741). Accessed March 3, 2025. Available at: https://accessgudid.nlm.nih.gov

