How to Order
FLEXBUMIN [ALBUMIN (HUMAN)] IS AVAILABLE IN THREE BAG SIZES WITH TWO CONCENTRATIONS TO HELP MEET YOUR CENTER’S NEEDS
FLEXBUMIN 25% [ALBUMIN (HUMAN)], US, 25% SOLUTION1
SIZE
EQUIVALENT
UNITS (EU)
ORDERING
CODE*
NDC
NUMBER (BOX/ CARTON)
NDC
NUMBER (BAG)
PACK
FACTOR
INDIVIDUAL BAG DIMENSIONS
BOX
DIMENSIONS
SHIPPING
WEIGHT
FLEXBUMIN 5% [ALBUMIN (HUMAN)], US, 5% SOLUTION2
SIZE
EQUIVALENT
UNITS (EU)
ORDERING
CODE*
NDC
NUMBER (BOX/ CARTON)
NDC
NUMBER (BAG)
PACK
FACTOR
INDIVIDUAL BAG DIMENSIONS
BOX
DIMENSIONS
SHIPPING
WEIGHT
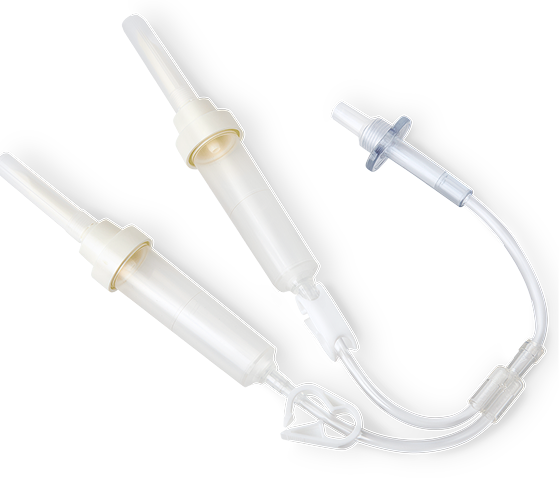
FLEXBUMIN 5% [ALBUMIN (HUMAN)] SOLUTION WITH Y-CONNECTOR SET
Y-connector sets enable dual infusion when large volumes of FLEXBUMIN 5% [Albumin (Human)] are required
SIZE
EQUIVALENT
UNITS (EU)
ORDERING
CODE*
NDC
NUMBER (BOX/ CARTON)
NDC
NUMBER (BAG)
PACK
FACTOR
INDIVIDUAL BAG DIMENSIONS
BOX
DIMENSIONS
SHIPPING
WEIGHT
*Product ordering code to be used only when ordering directly from Takeda.
†Includes the port length which is approximately 1.75".
‡Use this code to receive Y-connector sets at no charge with your order. You will receive 5 Y-connector sets per case (10 bags/case). Only available from Takeda.
Y-CONNECTOR SETS OFFERED BY TAKEDA:
Y-connector sets can be ordered with FLEXBUMIN 5% [Albumin (Human)], USP, 5% Solution at no additional charge.
WAYS TO ORDER FLEXBUMIN:
- Directly from your distributor
- Takeda e-commerce store by visiting Store.Takeda.com
- Directly from Takeda by calling 1-800-423-2090
For more information about FLEXBUMIN or for information on contracting options for your site of care, contact your Takeda Representative, or contact Takeda Customer Service by phone at 1-800-423-2090.

GET ANSWERS TO YOUR QUESTIONS.
Takeda has a dedicated and experienced hospital team of account managers who can provide a personal and tailored approach to address the needs of your system.
References
- FLEXBUMIN 25% [Albumin (Human)], USP, 25% Solution. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- FLEXBUMIN 5% [Albumin (Human)], USP, 5% Solution. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
